gammaCore Sapphire™ device
gammaCore™, a US FDA,TGA, Health Canada, UK CA and European CE Mark certified device, delivers non-invasive neuromodulation by stimulating the cervical branch of the vagus nerve. This rechargeable hand-held device includes:
- User selected intensity levels for personalised treatments.
- Up to 24 stimulations per day, so you can treat multiple attacks.
- 93 consecutive treatment days, enough treatments for 3 full months (treatment days may vary depending on model).
- Refill cards for your device are available to extend your treatment period.
- 36 month device option for chronic use.
How gammaCore™ works
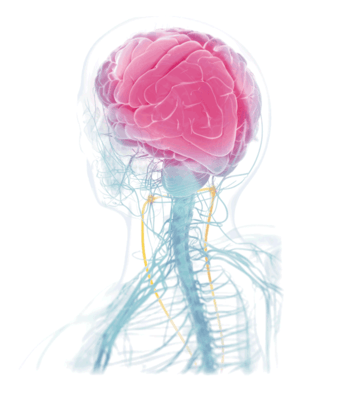
Unlike an implanted vagus nerve stimulator or other migraine and cluster headache treatments, the gammaCore™, a non-invasive vagus nerve stimulator (nVNS), activates the cervical branch of the vagus nerve, helping to prevent and relieve headache pain without pills, injections and medication-induced side effects.
As the longest cranial nerve in your body, part of the vagus nerve passes from the brain stem through the neck as it travels between the chest and abdomen. During treatments, gammaCore™ can be placed on either side of the neck where the vagus nerve is located and sends gentle electrical impulses through the skin. The electrical energy stimulates the vagus nerve to facilitate healing and balance.
Non-invasive and low risk
Very easy to use with many therapeutic benefits. No risk of overuse and can be used with other medications as needed.
Portable
gammaCore™ is a handheld, portable nVNS device that can be operated from your own home or during your daily activities.

Clinical practice
Demonstrated efficacy in multiple clinical trials. Well tolerated with less medication-like side effects; can be used up to 24 times per day.
Intended use
gammaCore™ is indicated for the treatment and/or prevention of primary headache (migraine, cluster headache and hemicrania continua) and medication overuse headache in adults.
Backed by research
Vagus nerve stimulation has been widely researched and studied. The gammaCore™ device has 7 different FDA clearances with robust scientific & clinical data to support its safe and effective use, including 7 randomised controlled trials with more than 30 mechanisms of action peer-reviewed papers, and over 40 peer-reviewed clinical papers. Read these papers for more information.
- Non-invasive neuromodulation of the cervical vagus nerve in rare primary headaches
- Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers
- Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study
- Vagus nerve stimulation for prevention of migraine: The multicenter, randomized, double-blind, sham-controlled PREMIUM II trial
- NICE Guideline on transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine

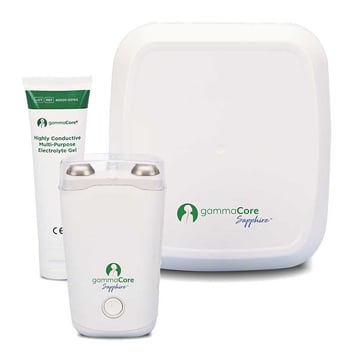
How to order your gammaCore Sapphire™
gammaCore Sapphire™ SLC
93-day Starter Kit
R 12,420.00 ZAR
gammaCore Sapphire™ SLC
93-day Refill Kit
R 12,420.00 ZAR
gammaCore Sapphire™ D
36-month Starter Kit
R 90,000.00 ZAR
Step 1

Step 2

Step 3

Once payment is received, we will arrange for the order to be delivered within 1-3 working days.
See gammaCore Sapphire™ device payment plan options here.
A guide to using my device
More information
Please visit our FAQs and troubleshooting page for more information about the gammaCore Sapphire™ device as well as other medical devices distributed by Byond Healthcare.
If you have any questions about how the gammaCore Sapphire™ could help you or patients in your practice, please feel free to contact Byond Healthcare.
For more information for healthcare providers on how to partner with Byond Healthcare, please complete our contact form.